How to: Burning Steel Wool
Here is a great how-to science experiment that you can try at home: burning fine steel wool with only a battery. The shock and awe that you get from the visuals is enough to get anyone excited. When you can explain the science, it’s even cooler. But, before we get to the experiment, you should know that there are some really cool artistic things you can do with it. Since we also teach filmmaking and photography, we thought you might first like the inspiration from this short video.
Burning Steel Wool (and Photography) Materials

Everything you need to make this happen can be purchased at Walmart, a hardware store or online. Here are the things you need with quick links to amazon if you want to simply add them to your shopping cart (and yes, buying them through here actually helps us out).
- Steel Wool: Pick 0, 00, 000, or 0000 grade:
- 9 volt battery:
- A small scale (to weigh it with afterwards)
- A kitchen whisk set: (If you want to do steel wool photography)
- A wire rope to spin it (Also for photographs)
- Fire Extinguisher
I added these amazon links incase you want to buy the needed materials. They are affiliate links, so if you buy them through the link, it will help us out. Good luck with your photography
How it Works
The first thing to understand is that steel wool is actually mostly iron (Fe). In fact, steel is an iron alloy: iron with about 2% carbon mixed in. For simplicity, lets just say it’s mostly iron.
We used a 9-volt battery to light the steel wool because the terminals are close together. Touching the battery to steel wool sends a current through the thin wire, and it heats up a lot (to about 700 degrees C). These temperatures cause the iron to react with the oxygen (O2) in the air and creates iron oxide (FeO2).
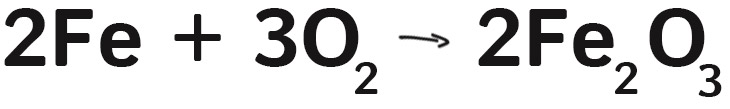
This reaction releases heat, heating up the next bit of iron and so on, causing a cascading reaction through the steel wool. Fluffing up the steel wool and spinning it increases the amount of oxygen available, speeding up the reaction and giving us the amazing display that we used for these photographs. Cool, huh?
Warnings
Now to warn you about the hazards. Be very careful with this. You are dealing very hot things and spreading them over a considerable distance. Try to always do it over water or concrete as they will catch things on fire, even when you’re trying to be careful. Plus, some people consider this littering as you always send out little chunks of steel. Do you best to clean it all up when you’re done.
And of course, they can also burn you. Make sure you always have:
- Eye protection
- Head protection
- A Fire Extinguisher
- Non-flammable clothing to cover your skin
- gloves
Steel Wool Science
Weigh the Steel Wool
The science of this is pretty cool. You’re basically creating a new substance—iron oxide—from iron and oxygen. After the reaction, the final product is actually heavier than the original steel wool. Who would have thought that burning something would make it weigh more? That’s pretty neat!
Another Science Video About Steel Wool
If you’re a parent or teacher and want to introduce your students/kids to this without actually doing it, I also recommend watching this science video we did.
Again, remember to be very careful with this. Good luck doing science and/or taking photographs with steel wool.
3 thoughts on “Steel Wool Science”
Comments are closed.






























































































Very very nice article. So interesting. Nice job.
very cool
super cool